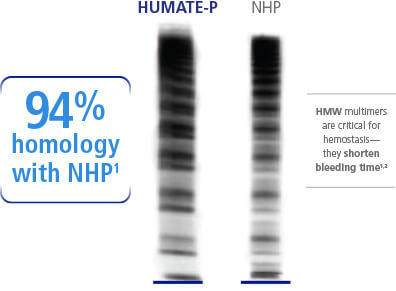
The multimeric pattern of HUMATE-P has been shown to very closely resemble that of normal human plasma
Like normal human plasma (NHP), HUMATE-P contains a high percentage of HMW-VWF multimers, and can correct the hemostatic defect in patients with VWD.
Preferred
HUMATE-P is the #1 prescribed VWF/FVIII concentrate treatment.3
- Extensive clinical experience
- Long record of success
HUMATE-P has been studied for more than 30 years and is preferred by more physicians for their patients. In fact, it is the most prescribed von Willebrand factor/factor FVIII (VWF/FVIII) replacement therapy indicated for von Willebrand disease (VWD) in:
- Infants
- Children
- Adolescents
- Adults
Proven
HUMATE-P provides reliable hemostatic control for all VWD types.
HUMATE-P is also proven effective across multiple types of bleeds, including:
-
All bleeding episodes, including spontaneous bleeding episodes (bleeding that occurs without an obvious cause) or after an injury, such as nosebleeds4
- 97% of patients overall (100% Type 1, 100% Type 2, 95% Type 3)
-
Trauma-induced bleeding episodes (bleeding caused by accidents or injuries)5
- 100% of urgent surgery patients
-
Bleeding occurring before and after surgery6
- 96% on day of surgery
- 100% Day 2 post-surgery, 100% Day 14 post-surgery)
Purified
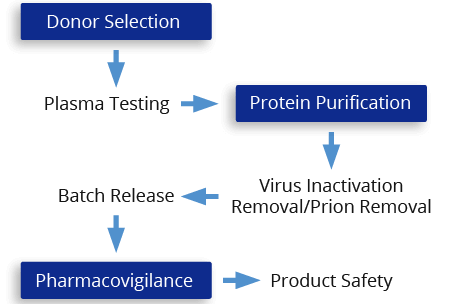
CSL Behring's Integrated Safety System helps the product meet high quality and safety standards, thus reducing the risk of virus transmissions. The risk of virus transmission cannot be completely eliminated.
CSL Behring is dedicated to meeting the most stringent international standards for plasma-product safety in accordance with guidelines from worldwide regulatory agencies.
The Integrated Safety System is designed to maximize virus inactivation and removal while maintaining biological integrity
HUMATE-P undergoes multiple steps during manufacturing to reduce the risk of virus transmission:
- Cryoprecipitation
- Al(OH)3 adsorption, glycine precipitation, and NaCl precipitation, studied in combination
- Pasteurization in aqueous solution for 10 hours at 60°C
- Lyophilization
The Power of Heat Treatment
This heat treatment step illustrated here has been proven to inactivate both enveloped and non-enveloped viruses.
References
- Metzner HJ, Hermentin P, Cuesta-Linker T, Langner S, Müller H-G, Friedbold J. Characterization of factor VIII/von Willebrand factor concentrates using a modified method of von Willebrand factor multimer analysis. Hemophilia. 1998;4(suppl 3):25-32.
- Budde U, Metzner HJ, Müller H-G. Comparative analysis and classification of von Willebrand factor/factor VIII concentrates: impact on treatment of patients with von Willebrand disease. Semin Thromb Hemost. 2006;32:626-635.
- Data on file. Available from CSL Behring as DOF HUM-002.
- Lillicrap D, Poon M-C, Walker I, Xie F, Schwartz BA, and members of the Association of Hemophilia Clinic Directors of Canada. Efficacy and safety of the factor VIII/von Willebrand factor concentrate, Haemate-P/HUMATE-P: ristocetin cofactor unit dosing in patients with von Willebrand disease. Thromb Haemost. 2002;87(2):224-230.
- Thompson AR, Gill JC, Ewenstein BM, Mueller-Velten G, Schwartz BA; HUMATE-P Study Group. Successful treatment for patients with von Willebrand disease undergoing urgent surgery using factor VIII/VWF concentration (HUMATE-P). Hemophilia. 2004;10(1):42-51.
- Lethagen S, Kyrle PA, Castaman G, Haertel S, Mannucci PM, for the Haemate P Surgical Study Group. Von Willebrand factor/factor VIII concentrate (Haemate P) dosing based on pharmacokinetics: a prospective multicenter trial in elective surgery. J Thromb Haemost. 2007;5(7):1420-1430.
