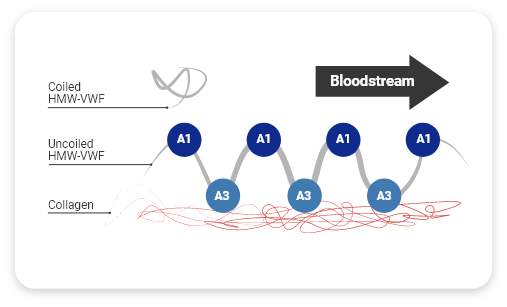
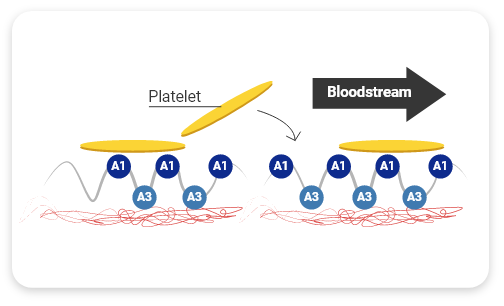
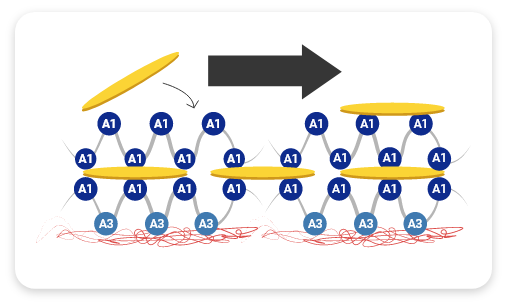
HMW-VWF multimers are associated with increased hemostatic activity and shortened bleeding time3
VWF replacement therapy is an important treatment for VWD. Restoring active VWF levels results in shortened bleeding time and correction of the coagulation abnormality.4
The multimeric pattern of HUMATE-P very closely resembles normal human plasma (NHP)
A densitometric analysis of various FVIII/VWF concentrates and normal human plasma (NHP) revealed that HUMATE-P contains a high percentage of HMW-VWF multimers—as does NHP—and is capable of correcting the hemostatic defect in patients with VWD.5
Densitometric analysis of HUMATE-P, NHP, and various other VWF agents
View an animated densitometric analysis comparing various VWF/FVIII concentrates and normal human plasma
HUMATE-P (represented in the graph by the blue line) had the highest content of HMW-VWF multimers among VWF products tested, and its multimer pattern was most like that of NHP
The multimer patterns of products A, G, H, and E (represented in the densitometric analysis by the red, green, orange, and yellow lines, respectively) differ from that of NHP
The multimeric pattern of HUMATE-P very closely resembles NHP
HMW multimers are critical for hemostasis— they shorten bleeding time2,4
94%
homology with NHP2

Effective bleed control with VWF+FVIII in a single therapy
Everyone who has VWD has a potential FVIII deficiency. Type 3 VWD, the most severe type, is associated with minimal FVIII, and people who have Type 2N VWD also have a FVIII deficit. In Type 1 and the other Type 2 VWD subtypes, FVIII levels can potentially be reduced. Administering VWF and FVIII together can help bleeding resolve quickly.6
Every patient with VWD has a potential FVIII deficiency6
1
2A
2B
2M
2N
3
One IU of VWF:Rco or FVIII in HUMATE-P is approximately equal to the amount of VWF:Rco of FVIII in 1.0 mL of NHP
The average ratio of VWF:RCo to FVIII
2.4 to 1

References
- Reininger AJ. Function of von Willebrand factor in haemostasis and thrombosis. Haemophilia. 2008;14(Suppl 5):11-26.
- Stockschlaeder M, Schneppenheim R, Budde U. Update on von Willebrand factor multimers: focus on high-molecular-weight multimers and their role in hemostasis. Blood Coagul Fibrinolysis. 2013;25:206-216.
- Metzner HJ, Hermentin P, Cuesta-Linker T, Langner S, Müller H-G, Friedebold J. Characterization of factor VIII/von Willebrand factor concentrates using a modified method of von Willebrand factor multimer analysis. Haemophilia. 1998;4(Suppl 3):25-32.
- Peyvandi F, Kouides P, Turecek PL, Dow E, Berntorp E. Evolution of replacement therapy for von Willebrand disease: from plasma fraction to recombinant von Willebrand factor. Blood Reviews. 2019;38:10057. https://doi.org/10.1016/j.blre.2019.04.001. Accessed October 6, 2020.
- Budde U, Metzner HJ, Müller H-G. Comparative analysis and classification of von Willebrand factor/factor VIII concentrates: impact on treatment of patients with von Willebrand disease. Semin Thromb Hemost. 2006;32:626-635.
- Nichols WL, Jr., Chair. The diagnosis, evaluation, and management of von Willebrand disease. US Department of Health and Human Services. National Institutes of Health. National Heart, Lung, and Blood Institute. NIH Publication No. 08-5832. December 2007.