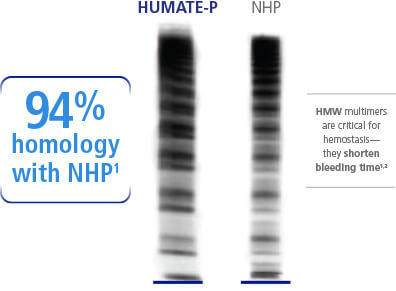
VWF Densitometric Analysis
In a densitometric analysis of various FVIII/VWF concentrates, HUMATE-P has been shown to have the highest content of HMW-VWF multimers, represented in the graph by the blue line. The multimer pattern of HUMATE-P is most like that of normal human plasma, which is represented by the black line. In contrast, the multimer patterns of products A, G, H, and E, represented in the densitometric analysis by the red, green, orange, and yellow lines, respectively, differ markedly from that of normal human plasma. Specifically, HUMATE-P contains 94% of the corresponding bands in normal human plasma.
The densitometric analysis shows that, like normal human plasma, HUMATE-P contains a high percentage of high molecular weight von Willebrand factor (HMW-VWF) multimers, and it is capable of correcting the hemostatic defect in patients with VWD.
View an animated densitometric analysis comparing various VWF/FVIII concentrates and normal human plasma
References
- Metzner HJ, Hermentin P, Cuesta-Linker T, Langner S, Müller H-G, Friedbold J. Characterization of factor VIII/von Willebrand factor concentrates using a modified method of von Willebrand factor multimer analysis. Hemophilia. 1998;4(suppl 3):25-32.
- Budde U, Metzner HJ, Müller H-G. Comparative analysis and classification of von Willebrand factor/factor VIII concentrates: impact on treatment of patients with von Willebrand disease. Semin Thromb Hemost. 2006;32:626-635.
