We can trace von Willebrand’s back to my great grandfather on my dad’s side. My father had 4 daughters and a son. All 5 of us have a bleeding disorder.”
—Roberta, person treating VWD with HUMATE-P
In most cases, VWD is inherited, meaning it is passed down through the genes. Each type of VWD is inherited differently. A man or woman with VWD has at least a 50% chance of passing on the VWD gene to his or her child. On rare occasions, people who didn’t inherit an abnormal gene can develop VWD. This condition, known as acquired von Willebrand syndrome, is caused by an underlying medical condition.
A person who has VWD has a 1-in-2 chance of passing on the gene to his or her children.

Adapted from Hemophilia Federation of America.
We can trace von Willebrand’s back to my great grandfather on my dad’s side. My father had 4 daughters and a son. All 5 of us have a bleeding disorder.”
—Roberta, person treating VWD with HUMATE-P

Low amounts of
VWF. Most common
type of VWD

VWF does not function
properly. Four subtypes
2A, 2B, 2M, 2N

Extremely low levels of
VWF or no VWF. Most
severe type of VWD
Everyone’s VWD experience is unique. Your symptoms and their severity may differ from the description of your type of VWD.
A person who has VWD may experience symptoms such as bruising for no apparent reason, spontaneous nosebleeds, or prolonged bleeding after an injury, childbirth, dental procedure, or surgery. Women who have VWD may experience heavy or prolonged menstrual periods—and they may think their heavy bleeding is normal because it runs in the family.
VWD bleeding may be mild, moderate, or severe. Some people may not have any symptoms at all.

Easy bruising

Frequent or prolonged nosebleeds

Heavy or prolonged menstrual periods

Prolonged bleeding in childbirth

Prolonged bleeding after surgery

Prolonged bleeding during dental procedures
It all made sense once we were diagnosed, but VWD is not something I would have ever thought of.”
—Bekah, person treating VWD with HUMATE-P
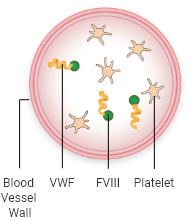
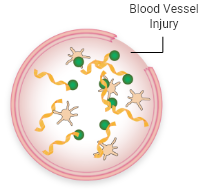
Von Willebrand factor (VWF) plays a key role in forming blood clots. The body forms blood clots to protect against excessive bleeding. The process, known as coagulation, is a sequence of steps that lead to clot formation. Coagulation involves several clotting factors and is triggered when a blood vessel is injured.

Platelets:
blood cells that gather at the site of blood vessel injury

Factor VIII (FVIII):
a protein in the blood that is needed for clot formation

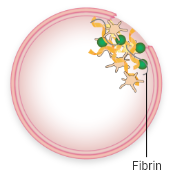
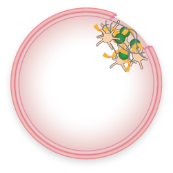
Fibrin:
a tough web-like protein that holds platelets together and keeps the clot in place
When activated, VWF attracts platelets and binds them to the injured blood vessel wall. VWF also carries FVIII, a key component of the coagulation cascade, to the blood vessel injury. This process involves the interaction of multiple clotting factors and results in the formation of a fibrin blood clot.
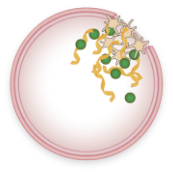
VWF bound to FVIII and platelets circulate in the blood
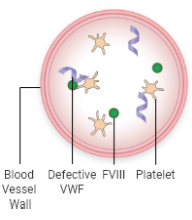
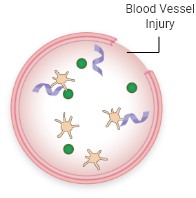
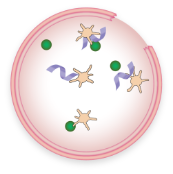
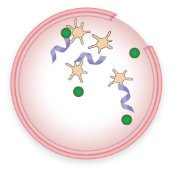
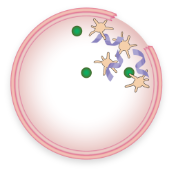
FVIII and VWF levels or activity are low
VWF carries FVIII and releases it at the site of injury
Less FVIII is brought to the site of injury
VWF attracts platelets to the injury
Platelets stick together, forming a “plug”
Fewer platelets are attracted to the injury
Little or no “plug” is formed
Activated FVIII and other clotting factors in the coagulation cascade interact and lead to fibrin formation
Less FVIII is available to interact with other clotting factors, so less fibrin is formed
Fibrin strands hold the platelets together, creating a stable blood clot
A stable blood clot is not formed, allowing bleeding to continue
A detailed discussion can help you and your doctor find the best treatment for your bleeding disorder. The HUMATE-P Doctor Discussion Guide can help you start the conversation.
Your email address will be used only for the purpose of sending the Doctor Discussion Guide and will not be stored.
Want to know more about VWD?